Acid Base Equilibrium Practice Test
Breaks stable hydrogen bonds. Ap Chemistry Acid Base Equilibrium Practice Test and Practice Exam are the textbook and this website is the perfect place to practice for the AP Chemistry exam.
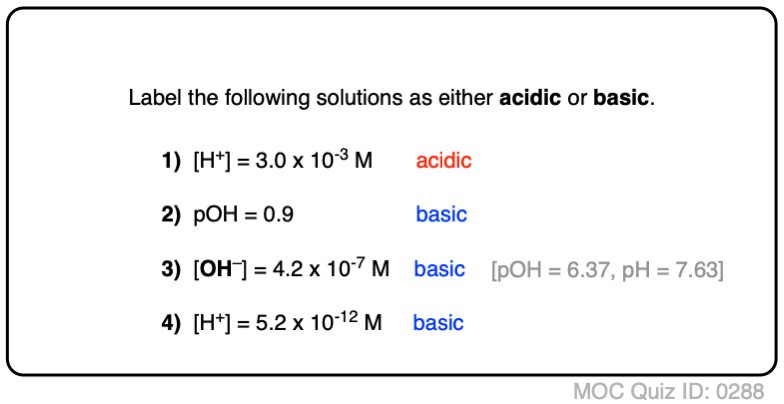
Acid Base Practice Problems Master Organic Chemistry
Forms stable hydrogen bonds.
. A buffer system resists changes to pH when small amounts of acid or base are added to it. Kab -14for a conjugate acidbase pair Quadratic Equation. In the BrønstedLowry definition of acids and bases a base _____ a.
Organic Chemistry Acid Base Equilibrium Practice QuestionsNeed help with Orgo. Chemistry of buffers and buffers in our blood. The concentrations stay steady once equilibrium is established.
The buffer region is marked by the letter __. 01 mol dm 3 NH 4 OH and 005 mol dm 3 HCl. Which statement about buffer systems is not correct.
A buffer system is made by mixing a weak acid with a weak base. Select your preferences below and click Start to. Here we have a weak base and so we will need its salt.
A buffer system may contain a weak acid and its conjugate base. The acid has one more proton than. Up to 24 cash back RTD Chemistry 30 Unit 2 Equilibrium Focussing on Acids and Bases 7 NR 5 Weak acid-strong base titration curve.
In option c concentration of strong acid is less than base. A technician has been given two unlabelled basic solutions. A buffer system may contain a weak base and its conjugate acid.
SCH 4UI UNIT 4. Brønsted and Lowry c. Test prep MCAT Foundation 5.
Download my free guide 10 Secrets to A. A Its a dynamic equilibrium. An electron-pair acceptor is a a.
Whose definition of acids and bases emphasizes the role of protons. Practice Test 161 pg 1 of 3 Acid Base Equilibrium The Ka of hydrazoic acid HN3 is 19 x 10-5 at 250 C. Use the following information to answer this question.
CHEMICAL SYSTEMS AND EQUILIBRIUM CHAPTER 8 ACID-BASE EQUILIBRIUM LESSON 81. In the BrønstedLowry definition of acids and bases an acid _____ a. X a bbac 2 24 1.
Under the premise of a chemical equilibrium which of the following can be presumed. Acids Bases and Conjugates Miscellaneous 1. Which of the following is false about a system at equilibrium.
01 mol dm 3 CH 4 COONa and 01 mol dm 3 NaOH. Look Im not trying to be mean but they are some of the most annoying and. Practice calculations involving solutions of weak acids and bases in this set of free practice questions designed for AP Chemistry students.
Practice Study Guide Course Acids Bases Equilibrium Chapter Test Prep Plan - Take a practice test Acids Bases Equilibrium Chapter Exam. Ionisation Constant of AcidBase Salt Recommended MCQs - 194 Questions Equilibrium Chemistry Practice questions MCQs Past Year Questions PYQs NCERT Questions Question Bank Class 11 and Class 12 Questions NCERT Exemplar Questions and PDF Questions with answers solutions explanations NCERT reference and difficulty level. One is a weak base and one is a strong base but they have the same pH.
Ka and acid strength. For a buffer we need to have a weak baseacid and its salt. None of these answers are correct.
If an acid is combined with a base of equal strength the result will most likely be. Is a proton acceptor. SCH 4U Acid Base Equilibrium Practice Test Answers can be found on my website 1.
Reactants and products are constantly going back and forth b Its a steady state. Two species related to each other through the gain or loss of a proton. Instead were using CARIO to determine which acids and bases are stronger and predicting the reaction direction accordingly.
Chemical processes Acidbase equilibria. A model of acid-base behavior based on proton transfer in which an acid and a base are defined respectively as a species that donates a proton and one that accepts a proton. Is a proton donor.
What is the pH of a 035 M aqueous solution of HN3. The state of equilibrium can be maintained over time as long as all. Strong bases are a.
A 0500 mole sample of which compound below when placed in water gives the lowest concentration of NO2-1 ions. Science Courses OSAT Chemistry CEOE 004. THE NATURE OF ACID-BASE EQUILIBRIA P528 549 TOPICS Brønsted Lowry Theory Reversible Acid-Base Reactions The Autoionization of Water Strong Acids and Strong Bases Hydrogen Ion Concentration and pH pOH and p K w.
So we will have both a weak base and its salt. View acid_test_practicedoc from CHEM 115 at Mount Royal University. This online quiz is intended to give you extra practice in calculations involving weak acids and bases including K a K b pH and ion and solution concentrations.
Buffer systems in the. Impossible to tell without testing the pH. This free online practice test is an effective way to see how well you know your AP course material.
This video walks you through a few practice questions where youre asked to determine the position of equilibrium for acid-base reactions WITHOUT using PKA values.
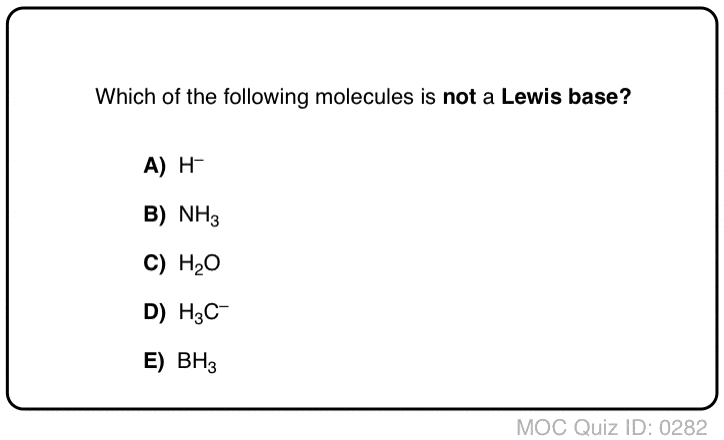
Acid Base Practice Problems Master Organic Chemistry

Acid Base Equilibrium Practice Organic Chemistry Youtube

Acid Base Questions Practice Khan Academy

Acid Base Equilibrium Organic Chemistry Practice Questions
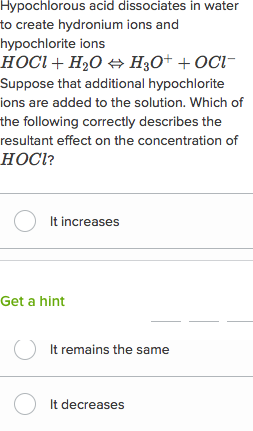
Comments
Post a Comment